For families living with complex lymphatic anomalies (CLAs), medical care often feels like trial and error. Treatments that work for some don’t always work for others, and doctors still lack clear answers about why these diseases behave the way they do. But new research from Dr. William (Bill) Polacheck and his team at the University of North Carolina at Chapel Hill offers a promising step forward.
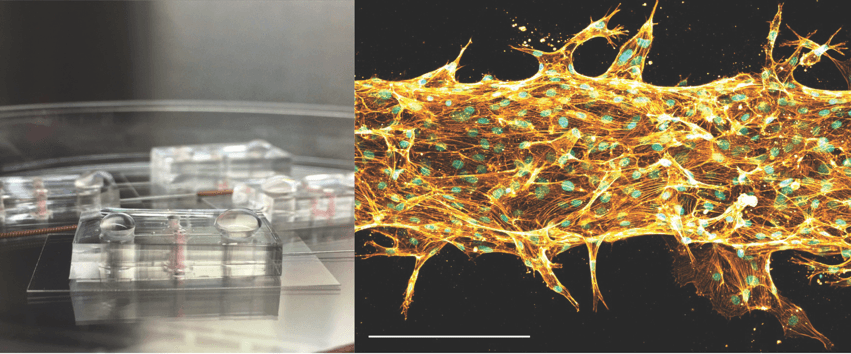
Image: Microfluidic CLA “chips” – each one is about the size of a quarter. On the right is an image of cells within the chip patterned to form a vessel-like tube that is about the width of a human hair (scale bar is 0.15 mm).
Building “Mini Vessels” in the Lab
Dr. Polacheck is developing tiny models of blood and lymphatic vessels—called microfluidic models—that can mimic what happens in the body. By growing cells inside 3D “chips,” researchers can watch how vessels form, how they leak, and how they respond to changes like blood flow.
For patients, this means scientists now have a tool to study CLAs in ways that were never possible before. Instead of relying only on animal models or patient biopsies, these lab-built vessels allow researchers to see how mutations linked to CLAs actually change cell behavior.
What They’ve Found So Far
One key discovery is that cells carrying common CLA-related mutations (like PIK3CA) don’t respond to blood flow the way healthy cells do. Normally, healthy vessels tighten up and strengthen when blood moves through them. But in these models, mutant cells stayed weak and “leaky,” leading to vessel changes that look a lot like the malformations patients experience.
The team also noticed that these mutant cells break down their surroundings faster, creating unstable, blobby vessel structures. Importantly, when treated with drugs like rapamycin or alpelisib—two therapies already being studied for CLAs—the vessels became more stable. This suggests the models could help test drugs more quickly and guide doctors toward more effective treatments.
Why This Matters for Patients
These models could be game-changers in several ways:
- Faster drug testing: Instead of waiting years for clinical trials, researchers can quickly screen potential therapies in the lab.
- Personalized care: In the future, doctors might use a patient’s own cells to create a mini-model of their vessels, then test which drugs work best for that individual.
- New treatment strategies: The research even hints at new ways to deliver medicine, like using nanoparticles that mutant cells absorb more readily than healthy ones.
Looking Ahead
Dr. Polacheck’s group is now working with patient-derived cells, which will bring this research even closer to real-world impact. Their ultimate goal is to create reliable platforms that help doctors understand why CLAs behave as they do—and to find better, more targeted therapies for patients and families living with these rare conditions.
Research like this is possible thanks to collaboration between scientists, clinicians, and the rare disease community. It brings us closer to a future where patients don’t just manage their disease but benefit from treatments designed specifically for them.