Cell Signaling Pathways
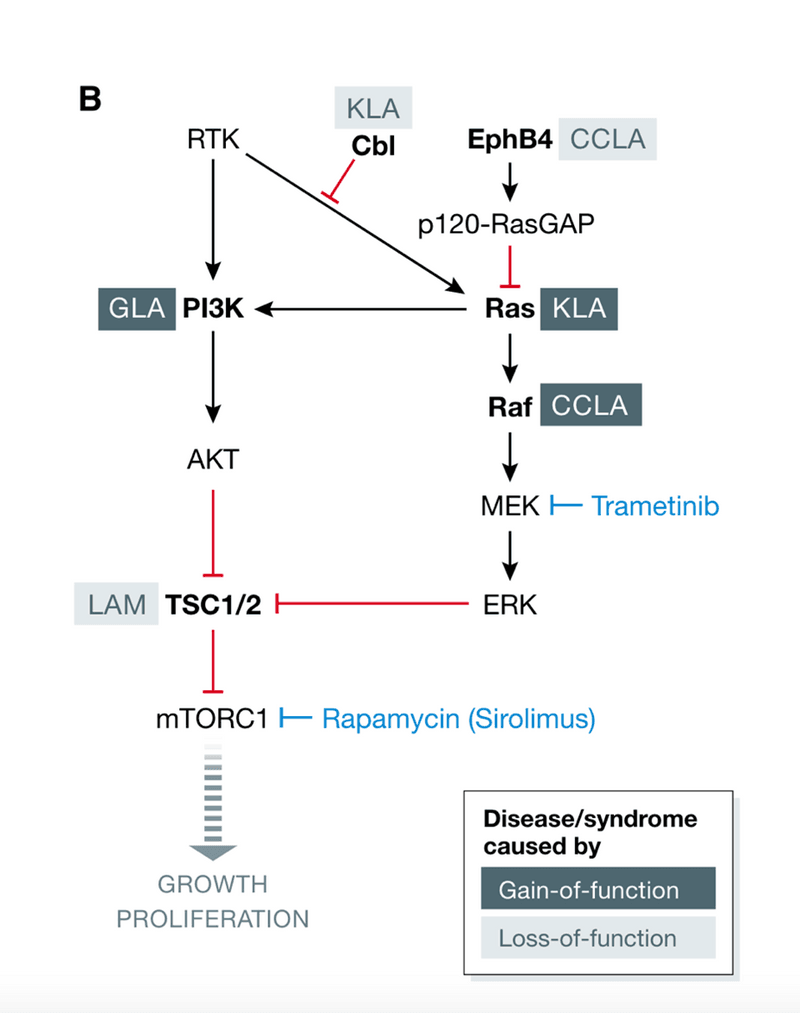
These genetic variants directly change the activities of intracellular signaling pathways, thereby influencing various downstream cellular processes. The pathways most affected in CLAs are the PI3K/AKT/mTOR and RAS/MAPK signaling pathways.
PI3K/AKT/mTOR pathway:
PI3K-Akt-mTOR pathway is an intracellular signal transduction pathway that promotes metabolism, proliferation, cell survival, growth, and angiogenesis in response to extracellular signals. It is commonly called the "anti-apoptosis pathway" because it prevents cell death.
Activation of PI3K typically occurs because of direct stimulation by the regulatory subunit bound to an activated receptor or indirectly by an adaptor molecule such as the insulin receptor substrate (IRS) proteins. Activation of PI3K converts PIP2 into PIP3. The second messenger PIP3 further stimulates protein kinase D (PDK), which activates Akt, starting the PI3K-Akt-mTOR signaling pathway that regulates cell proliferation. PI3K can also be activated by a GTP-bound RAS protein.
The general cellular impact of activating the PI3K-AKT-mTOR pathway is to stimulate cell growth and proliferation. Over-activation of this signaling pathway can overstimulate cells resulting in abnormal/excessive cell proliferation. Abnormalities in the PI3K pathway are common in cancer and lymphatic anomalies. PIK3CA-Related Overgrowth Spectrum (PROS) is a group of diverse overgrowth disorders caused by activating PIK3CA mutations. There are 13 disorders recognized as PROS including Klippel-Trenaunay syndrome (KTS), CLOVES (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal/spinal anomalies), and Proteus syndrome. (Reference 4)
The RAS/MAPK pathway:
The Ras-Raf-MEK-ERK signaling pathway regulates cell proliferation, differentiation, and survival. Overexpression and overactivation of members within the signaling cascade cause many human syndromes and diseases, including cancer.
The master regulator of the classical MAPK cascade is the Ras protein, which is encoded by three related genes, NRas, HRas, and KRas. Normally, Ras combines with GDP, which is in an inactive state. When Ras protein is released from Ras/GDP and binds to GTP, Ras is activated. Active Ras binds and activates Raf. Activated Raf phosphorylates and activates MEK1/2. MEK 1/2 phosphorylates ERK1/2 to activate signaling. Activation of ERK1/2 leads to its translocation into the nucleus and activates the expression of many downstream genes to promote cell proliferation and differentiation. In addition, activated Ras can directly bind and activate PI3K.
Syndromes resulting from genetic changes in the RAS/MPK pathway are called “RASopathies”. RASopathies include other more common disorders such as Noonan syndrome, Costello Syndrome, and Neurofibromatosis. (Reference 5)
Diseases Considered CLAs
Generalized Lymphatic Anomaly (GLA)
Formerly known as Lymphangiomatosis, is characterized by lymphatic malformations involving soft tissues, bones, and organs such as the spleen with disease present in multiple locations in the body. GLA may present at birth but is more frequently identified in childhood or young adulthood. GLA can cause pericardial, pleural (Figures 5, 6), or peritoneal effusions. It can also cause protein-losing enteropathy and leukopenia. Bone disease is commonly seen, involving multiple bones of the axial and appendicular skeleton. The ribs are the most common site of involvement in GLA, followed by the spine. Bone involvement in GLA does not typically include cortical bone and rarely results in progressive bone disappearance/loss. Fractures resulting from bone disease are uncommon. Activating somatic variants, or mutations, in the PIK3CA gene are thought to cause GLA. (Reference 6) PIK3CA variants are also found in other diseases, such as cancer and PIK3CA-related overgrowth spectrum (PROS).
Gorham-Stout Disease (GSD)
Is also called vanishing bone disease, is characterized by progressive loss of the cortex of the bone. GSD can progress rapidly but can also spontaneously stabilize. It typically extends beyond the original site of disease, involving nearby bones, and is much more common in the axial skeleton. Migration of lymphatic endothelial cells (LECs) into bone from soft tissue and their proliferation and expansion cause bone loss. Symptoms caused by GSD vary depending on the extent of the loss of cortical bone (Figure 7) and its location in the body. Pathologic fractures, the buildup of pericardial and pleural effusions (Figure 8) secondary to rib involvement, leaks of cerebral spinal fluid (CSF) resulting from skull-based damage, and neurologic symptoms including paralysis have all been reported. Activating somatic KRAS gene variants, or mutations, are thought to cause GSD. KRAS variants have also been seen in other diseases such as cancer and other vascular anomalies. (Reference 7)
Kaposiform Lymphangiomatosis (KLA)
Is a complex lymphatic anomaly with features of both neoplasia and malformation. KLA has characteristics that overlap with both GLA and CCLA, although the disease is often more aggressive. Symptoms are dependent on the location and severity of disease. Chest involvement is common in KLA. Affected tissues include the thoracic cavity, including the mediastinum, lung, and heart, and may be associated with pleural (Figure 9) and pericardial effusions. (Reference 8) Unique pathological features of KLA include abnormal lymphatic channels surrounded by spindle shaped LECs, rapid and progressive growth, and consumptive coagulopathy and hemorrhage. Hemorrhage, even in the absence of coagulopathy, can be a useful diagnostic characteristic. Markers to help diagnose KLA include elevated blood levels of angiopoietin 2 (Ang2), a protein involved in endothelial cell growth and function. Activating somatic variants, or mutations, in NRAS, CBL, or HRAS are thought to cause KLA.
Central Conducting Lymphatic Anomaly (CCLA)
Is characterized by dilated and dysfunctional lymphatic vessels in the torso leading to the backflow of lymphatic fluid into tissues. The dysfunction, in part, results from the abnormal lymphatic valves in the large vessels and dysplasia or hypoplasia of the thoracic duct resulting in chylous reflux, valve incompetence, and lymphatic leak (Figure 11). Patients often present with chylous effusions, ascites, protein-losing enteropathy, and swelling in the legs and feet. Both inherited (germline) variants in EPHB4 and MDFIC and somatic variants in ARAF, KRAS, and BRAF genes, may cause CCLA. (Reference 6) Syndromes in which CCLA was observed most frequently included RASopathies (both germline and mosaic), chromosomal abnormalities (e.g., trisomy 21), PIEZO1 lymphatic dysplasia, and metabolic disorders, confirming the involvement of RAS/MAPK signaling pathway.(Reference 9) Given the genetic variation in CCLA, genotype-phenotype correlations will be essential to identify subcategories under the general diagnosis of CCLA.






